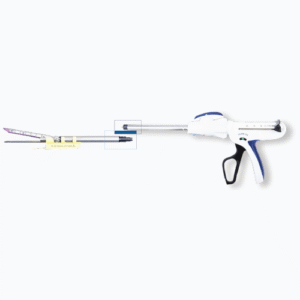
Models
| Model | Shaft Length (mm) | Anvil Length (mm) |
|---|---|---|
| FNAS60 | 280 | 60 |
| FNAM60 | 340 | 60 |
| FNAL60 | 440 | 60 |
| FNAS45 | 280 | 45 |
| FNAM45 | 340 | 45 |
| FNAL45 | 440 | 45 |
| FNAS30 | 280 | 30 |
| FNAM30 | 340 | 30 |
| FNAL30 | 440 | 30 |
| 📌 General Information | |
|---|---|
| Quality Management System | ISO 13485:2016 |
| Certificate | TÜV Rheinland LGA Products GmbH |
| Manufacturer | Fengh Medical Co., Ltd. D3 No.6, Dongsheng West Road, Jiangyin National High-tech Zone, Jiangsu, China |
| Scope of Application | The device is intended for performing transection, resection, and anastomosis in general, gynecological, urological, thoracic, and pediatric surgeries. It is also suitable for transection and resection of liver parenchyma, pancreas, kidney, and spleen. |
| Single Use | ✔ Yes |
| ⚙️ Technical Parameters | |
|---|---|
| Number of Drives | 15 |
| Jaw Articulation Angle | >110° |
| Jaw Articulation | Electric (Powered) |
| Jaw Movement | Electric (Powered) |
| Needle Closure | Motorized |
| Trocar Compatibility | Compatible with all 12 mm trocar refills |
| Technologies | Anti-slip Fabric Technology Triple Point Line Technology |
| 🔧 Materials | |
|---|---|
| Staple | Ti-3AL-2.5V |
| Anvil | Stainless SUS420 / SUS630 |
| Knife | Stainless SUS440 |
| Reload Housing | LCP |
| 📦 Packaging and Compliance | |
|---|---|
| Sterility | Sterile |
| Sterilization Method | Radiation (shelf life 3 years) |
| Packaging |
1 unit per package Primary packaging: Tyvek Paper + PETG Blister Secondary packaging: Cardboard box |
| Storage Conditions | Store in a ventilated, dry, non-corrosive environment. Avoid extrusion, abrasion, and dropping. |
| Biocompatibility | GB/T 16886 |
| Corrosion Test | YY/T 0149-2006 |